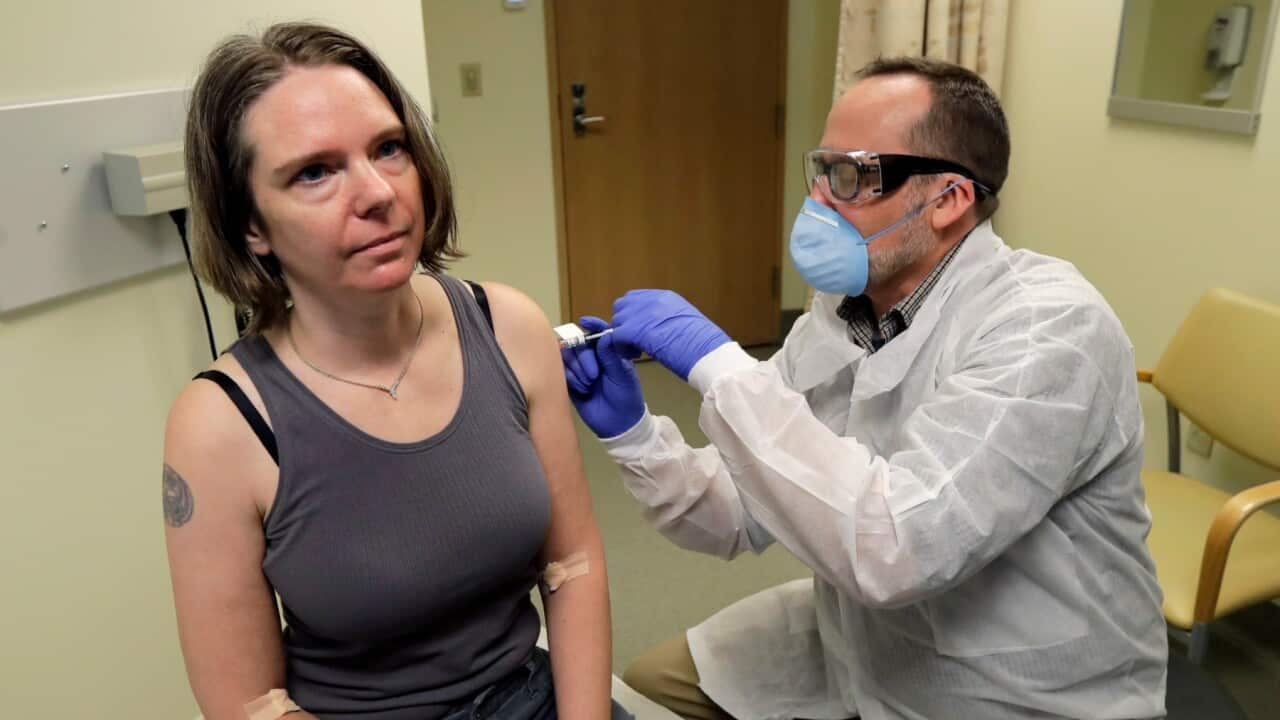
The Cambridge, Massachusetts-based company said the vaccine candidate, mRNA-1273, appeared to produce an immune response in eight people who received it similar to that seen in people convalescing from the virus.
"These interim Phase 1 data, while early, demonstrate that vaccination with mRNA-1273 elicits an immune response of the magnitude caused by natural infection," Moderna's chief medical officer Tal Zaks said.
Moderna, which was founded nine years ago, said the vaccine "was generally safe and well tolerated" and that patients suffered no more than redness or soreness from the shots.
In a conference call, Moderna chief executive Stephane Bancel said the preliminary tests inspired confidence that mRNA-1273 has "a high probability to provide protection" against the virus.
Separate tests performed on mice showed that the vaccine prevented the virus from replicating in their lungs, according to the company.
The US government has invested nearly half a billion dollars in the development of Moderna's vaccine candidate.
It is being developed in a partnership with the National Institute of Allergy and Infectious Disease headed by Dr Anthony Fauci and the clinical test was carried out by the National Institutes of Health.
Three groups of 15 patients aged 18 to 55 received three different doses of the vaccine in the Phase 1 test, the complete results of which are not yet known.
The Phase 2 trial, with 600 subjects, has already received the green light from the US Food and Drug Administration and Moderna said they should begin this quarter.
A Phase 3 trial, the largest and most important to validate the efficacy of a vaccine, should begin in July.