

Feature
Chris was using a sunscreen while he recovered from throat cancer. Then it was recalled
More than 20 sunscreen products have been taken off Australian shelves. For the most vulnerable, it's prompted "sunscreen anxiety".
Published
Updated
Chloe Holt bought an Australian sunscreen for her husband Chris on the advice of a sales assistant, who told her it was one of the "safest, most reliable" on the market.
Her husband was in the early stages of recovering from throat cancer and needed the "highest level" of sun protection while his skin healed from radiotherapy and chemotherapy.
But she says she was "horrified and frightened" to learn that the product — Ultra Violette's Lean Screen SPF 50+ Mattifying Zinc Skinscreen — was pulled from shelves after it returned test results showing an SPF (Sun Protection Factor) level as low as 4.
Now, Holt wonders if her husband was protected at all during the three months he was using it.
"He was left unprotected when he needed strong protection most," she tells SBS News.

"[Chris's cancer specialists] confirmed that his skin is still extremely vulnerable and won't fully heal for about three years after radiotherapy — so this was the worst possible time for him to be left unprotected."
Holt says the couple, who are based in the UK, once considered Australian sunscreens to be the "gold standard", but they've now lost trust in them.
"They're always talked about as the gold standard, but this experience has completely changed that for me," she says.
It's completely shaken that trust and confidence — in all SPFs, to be perfectly honest.
TGA regulations 'potentially outdated'
In June, an investigation by consumer advocacy group Choice found 16 of 20 products from a range of popular sunscreen brands failed to meet their advertised SPF ratings.
Ultra Violette's Lean Screen was one of them — returning the lowest SPF level of 4. After the company conducted its own testing, returning SPF results ranging from 4 to 64, the product was voluntarily recalled.
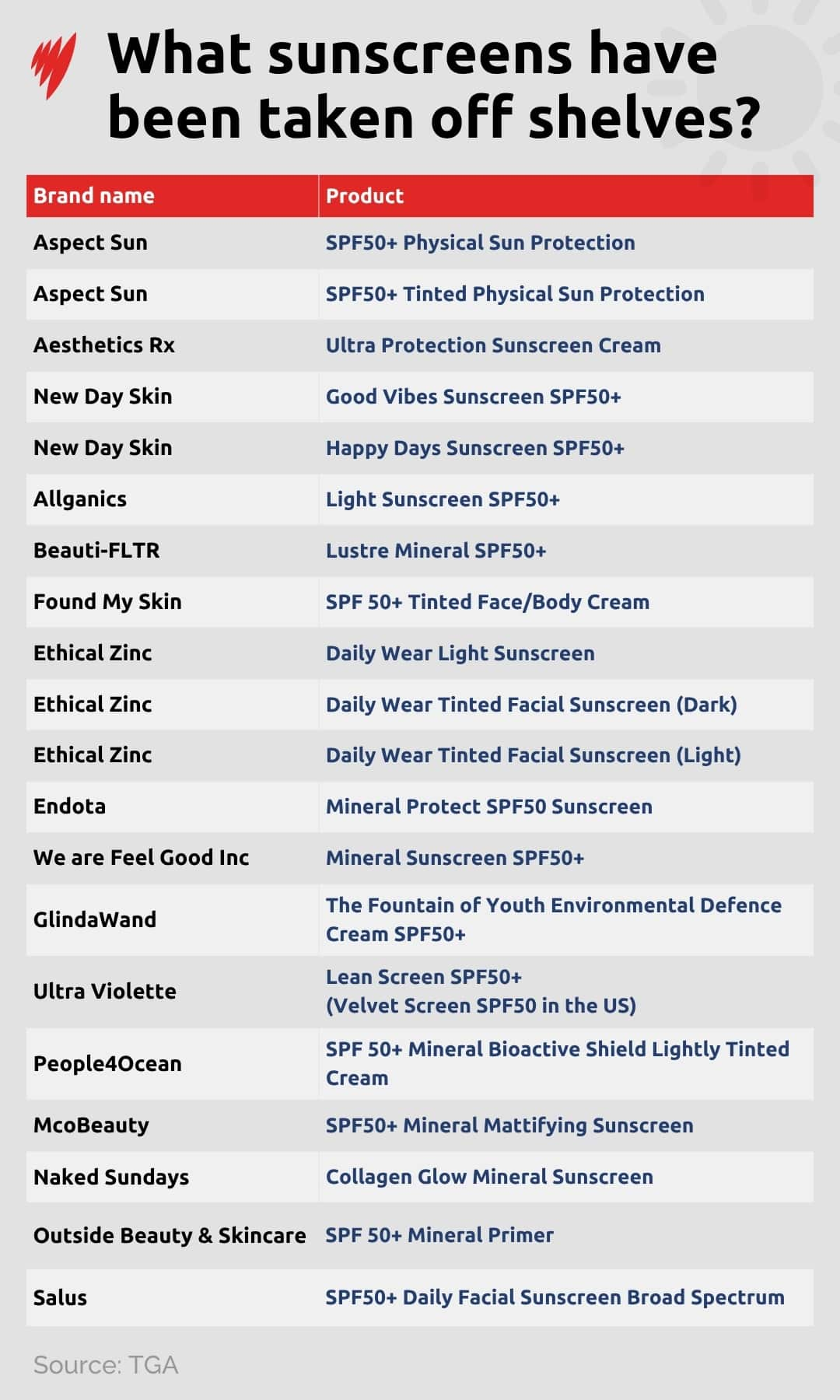
Following the findings, the Therapeutic Goods Administration (TGA) placed more than 20 sunscreen products — primarily physical sunscreens with the same base formulations — under review due to concerns they didn't meet their advertised SPF rating.
At the time of writing, 21 sunscreens have been either recalled or had sales paused in Australia, all of which are linked to a manufacturer called Wild Child Laboratories in Perth.
There's a reason why so many sunscreens have been caught up in so-called Sunscreen Gate.
In Australia, sunscreen brands don't actually manufacture their own sunscreen. That’s done by accredited manufacturers, such as Wild Child Laboratories. Those labs then use external testing facilities to validate their SPF ratings. Some brands might develop their own range, while others might take a base formulation, add small tweaks (such as fragrance) and then slap their label on it.
In a statement to SBS News, a spokesperson for Ultra Violette clarified that while "most formulations in our range are our own and have been developed by our in-house product development team", its Lean Screen product was one of the few formulations that was "manufacturer-owned".
The TGA's ongoing investigation found no manufacturing issues that could explain the variability in SPF testing results; instead saying it had "significant concerns" about the reliability of the testing facility used by Wild Child — Princeton Consumer Research Corp, a global research company headquartered in the US.
Dermatologist Dr Cara McDonald says Choice's findings aren't "surprising".
"The regulatory systems in Australia, although they're quite strict, they are potentially a little outdated and they're not necessarily reviewed and updated regularly," McDonald says.
She says that there's a "lack of transparency", especially in the manufacturing process.
"Most Australians don't realise that all these brands we're buying, they're not making their own sunscreen, they're actually just buying in a base," she says.

How SPF ratings are given
To sell a sunscreen product in Australia, brands must comply with strict TGA standards, which encompass SPF testing and broad-spectrum UVA testing, water resistance, and stability under various conditions.
But there are also limitations in testing techniques that even the TGA has recognised.
SPF ratings are determined through testing on humans (termed 'in vivo'). The process only measures UVB rays — the ones that cause sunburn — by monitoring how quickly skin starts to redden. Internationally, it's recognised — including by the TGA — that the testing is less reliable and comes with known variabilities.
The deeper penetrating and more dangerous UVA rays (synonymous with long-term skin damage and skin cancer) can only be tested in a lab ('in vitro'), which is considered to be a much more efficient and reliable method.
While the TGA mandates both UVA and UVB testing, McDonald says there is "ongoing work" to develop UVB testing methods in labs, thereby increasing the accuracy of results. But it's still not mandated.
A TGA spokesperson told SBS News that its regulation is "open and transparent" and that it is "currently considering" the implementation of new in vitro testing, acknowledging its reliability and "significant advantages".
The spokesperson also said the regulator is "investigating the viability of the TGA conducting in-house in vitro sunscreen testing".
"The TGA regularly reviews our legislation and policy to ensure they remain fit for purpose. If we consider that changes may be warranted, this is proposed and managed through a transparent consultative process."
For McDonald, Choice's findings and the subsequent recalls are proof that Australia's sunscreen regulations might not be as reliable as many thought they were.
"We've got this 'gold standard' label, not because the sunscreens are gold standard, but because everyone thought that this strict regulation that we have must mean our sunscreens are at least doing what they say they're doing," she says.
"This is where it's completely just ripped the floor out from underneath that."

'I had full faith'
For 35-year-old New Zealander Jess (who requested SBS News omit her surname), the realisation that the sunscreen she was using might not have been adequately protecting her was a hard pill to swallow.
After having a basal cell carcinoma — a type of skin cancer — cut out of her face at 29, she says she was "fastidious" with sunscreen use as she was at a higher risk of developing other skin cancers.
Like Holt's husband, she was also using Ultra Violette's Lean Screen.
It does make me feel quite anxious thinking about those two years I've used that sunscreen — and what the outcome of that will be for me.Jess
While she says she's always used extra protective layers, including hats, long sleeves and sunglasses, she still wonders whether her SPF was as protective as she believed it to be.
"Those two years where I thought I was protected: was I protected at all? I think about the damage that potentially happened in that time and it makes me feel a bit unwell.
"I just had full faith in it because I knew how much I was applying and reapplying."
In a statement to SBS News, a spokesperson for Ultra Violette said: "As a brand, we have always taken our responsibility to prioritise safety, protection and skin health above all else. That's why despite some tests that showed Lean Screen had moderate to high levels of SPF effectiveness we made the decision to recall the product in August."
"We are supportive of the TGA’s acknowledgement and move towards newer, more ethical testing methods. Ultra Violette has and will continue to, work directly with the TGA and through industry bodies like ACCORD to agitate for positive change and greater confidence in SPF claims."
The spokesperson also noted that the company was the first and "currently the only of the 16 products that failed Choice's test" to withdraw its product from the market and that it had voluntarily shared its formula with the TGA.
Several brands flagged in the study — including Banana Boat, Bondi Sands, and Cancer Council — said they retested their sunscreens following the investigation and found they matched the SPF 50+ claims.
The company has also offered customers refunds on its Lean Screen product.
While Jess says potential consequences are "worth a lot more than what a $50 tube of SPF is worth", she also acknowledged the brand has been "copping a lot of heat".
"There's a long list of SPFs from different companies that were not meeting the threshold and I'm seeing less criticism of them," she says.
'We don't need to panic'
McDonald says that while Choice's findings may be a sign that Australia's regulations have fallen behind, they also sound more alarming than they actually are.
An SPF rating of 30 is estimated to filter out 96.7 per cent of UVB radiation, while SPF 50 is estimated to filter out 98 per cent.
The TGA spokesperson added: "The difference in the amount of sun protection between an SPF of 25, 30 and 50 is relatively small, and all offer meaningful protection when used as part of a broader sun safety strategy."
So even if a sunscreen product has scored lower than its advertised rating claims, chances are customers are still getting pretty good coverage if they apply and reapply often, McDonald says.
The difference in the amount of sun protection between an SPF of 25, 30 and 50 is relatively small.Therapeutic Goods Administration
The problem is: most people don't.
Data from the Cancer Council found that only half of Australians are using three or more forms of sun protection (such as hat, sunglasses and long clothing) during peak UV times. Only two in five Australians use sunscreen on most days during late spring and summer, when daily sunscreen use is recommended.
Full coverage for an adult body will require about seven teaspoons of sunscreen every two hours, or more often after swimming or sweating — a lofty goal even for the most sun-conscious.
"Even though we don't need to panic about SPF20 versus 50, so to speak, we do need to realise that we're probably starting at a much lower SPF level than we think," McDonald says.
"[We] may end up more likely to get burnt and more likely to get UVA damage, which is going to cause pigmentation, ageing, inflammation and increase our risk of skin cancer without burning us."
Australian lupus community 'beyond anxious'
Angie Knaggs isn't sure if she can trust her sunscreen either.
The 42-year-old has lupus, a chronic autoimmune disease in which the immune system attacks the body's healthy tissues.
She says 'SunscreenGate' has "deeply affected" those in the Australian lupus community, as the disease causes sensitivity to sunlight and UV radiation.
According to the Johns Hopkins Lupus Center in the US, sun exposure can cause sunburn-like reactions, skin rashes, and flares in other parts of the body. Lupus also increases the risk of skin cancer from UV radiation.

Additionally, many medications that treat lupus can cause additional sun sensitivity, Knaggs says. This means that skin checks every six months, wearing Ultraviolet Protective Factor (UPF) clothing, and carrying an umbrella on sunny days are all pretty standard fare.
But after using two products being reviewed by the TGA — Lean Screen and Naked Sundays Collagen Glow Mineral — she's now been advised by medical experts to go for a skin check every three months, "as the risk of skin cancer lesions has increased".
"The entire community is beyond anxious — most are heading in for deep skin cancer checks and are left wondering what long-term damage these products have actually caused us," Knaggs says.
I'm extremely anxious about what this has caused long-term to my organs.Dr Angie Knaggs
Naked Sundays' product was not included in the Choice investigation, but shares the same base formulation as Lean Screen. Preliminary testing of the base formulation by the TGA estimates that the formulation is unlikely to have an SPF greater than 21, and may be as low as 4.
In a message sent to Knaggs in August, a Naked Sundays representative said there was "no risk [of] any damage — the product still adheres to current TGA guidelines".
In a statement provided to SBS News, Naked Sundays and its stockist MECCA said the companies had jointly "chosen" to remove the product from sale, "while Naked Sundays awaits independent re-testing results".
"This is not a recall; this is a proactive step that we have taken in partnership with Naked Sundays," the statement read.
Physical sunscreens scored worst, but only option for some
Most of the sunscreens placed under review by the TGA are physical sunscreens (also known as zinc or mineral).
In the Choice investigation, physical zinc (or mineral) sunscreens overall performed more poorly compared to chemical sunscreens.
"There were some chemical sunscreens in the lower group as well, but the majority of the poorly performing sunscreens are physical sunscreens," McDonald says.
While chemical sunscreens absorb into the skin and "sponge" up any UV, physical sunscreens act like a layer of film.
McDonald says there are plenty of drawbacks to physical sunscreens: They're more likely to be rubbed off, they aren't as stable as chemical sunscreens, and brands will often need to modify base formulas to offset their thickness and white cast (residue).
The TGA says that alterations to the base formulation "could increase or decrease the SPF of a sunscreen".

But for some consumers, physical sunscreens are the only option they have.
SBS News spoke to a 30-year-old from Victoria, who asked to remain anonymous, who is allergic to the chemical butyl methoxydibenzoylmethane (BMDM) found in many sunscreens.
She says her allergy has made it "hard" for her to access many sunscreens and that she was using the Naked Sundays product. Now the findings — and an inaccessible market — have put her in a "tight spot".
"I'm forced to turn to mineral-based products," she says.
"However, now it's hard to tell which ones are actually effective products or real sunscreens."
Another 30-year-old Sydneysider told SBS News they were "horrified" after seeing that two products they had used in the past — Ultra Violette's Lean Screen and Naked Sundays' Collagen Glow Mineral — were pulled from shelves.
She says she's now experiencing "sunscreen anxiety".
"I have lost confidence in my skin protection," she says.
"Right now, I don't trust the sunscreen in Australia."
A 'wake-up call' to the TGA
Professor Mark Morgan, a professor of general practice at Bond University and chair of The Royal Australian College of General Practitioners' (RACGP) expert committee, says it's understandable that some consumers are feeling anxious following the recalls.
"It's understandably worrying to people who have been using these sunscreens and imagining that it's been more protective than it has been in reality," Morgan says.
"Australia has a very high rate of skin cancer and people need to be able to rely on the sunscreen products that they use to help protect themselves from the harmful effects of the sun."
It's understandably worrying to people who have been using these sunscreens.Professor Mark Morgan, Bond University
He's calling on the TGA to release a "trust list of verified sunscreens" as soon as possible.
"It's really important that we have a reliable use of sunscreen and that we know which ones work and which ones to buy," he says.
The TGA spokesperson said "There are over 950 sunscreens currently included in the ARTG [Australian Register of Therapeutic Goods], which is a publicly available searchable database.
"Consumers can tell if sunscreens are included in the ARTG by looking for the ‘AUST L’ number on the product’s label."
Morgan cautions against losing faith in the TGA, saying: "I think we can trust the TGA, but it's been an important wake-up call where there are gaps in the TGA's processes or in its ability to monitor the safety of health products."
McDonald also hopes for a total review by the federal regulator: "Hopefully in the near future, we're going to see TGA do a review of all the sunscreens and all these brands will get retested."
She's also seen more anxiety about which products to pick and brands to trust.
"One of the things I've heard most is the concern regarding the Cancer Council sunscreens and people feeling so let down and disappointed by their results," she says.
Of the four Cancer Council sunscreens tested by Choice, just one matched its SPF50+ claims — Cancer Council Kids Sunscreen 50+. Others returned SPF ratings of 24, 27 and 33.
"People saw that as the epitome of sunscreen. It's the Cancer Council — their job is to protect us from cancer," McDonald says.
One of the things I've heard most is the concern regarding the Cancer Council sunscreens and people feeling so let down and disappointed by their results.Dr Cara McDonald
A Cancer Council spokesperson told SBS News that the TGA decision to recall sunscreens demonstrates "strong and robust sunscreen regulation, vital for maintaining consumer trust and confidence in sunscreens".
"We understand that the CHOICE investigation and updates from the TGA will have caused some concern, particularly for Australians who are at higher risk of skin cancer.
"All Cancer Council products named in the CHOICE report were retested, and these test results have been provided to the TGA. Based on those results, the regulator confirmed there is no safety concern with our sunscreens and no reason to withdraw any products at this time."
While Knaggs's trust in the TGA hasn't been hindered, she's still left with simmering anxiety.
"It's been an excellent wake-up call that we cannot rely on sunscreen and hats alone. "There's a total distrust [in physical sunscreens]," she says.
"The question we sit with now is — what sunscreen is safe?"
Clarification: This story has been updated to include an additional statement from Ultra Violette regarding its product formulations, noting that its Lean Screen product was "manufacturer-owned".
For the latest from SBS News, download our app and subscribe to our newsletter.


